Sickle Cell Task Force raises public awareness of sickle cell disease and sickle cell trait.
Texas Health and Safety Code, Chapter 52, directs the Texas Health and Human Services (HHSC) Executive Commissioner to establish and maintain a task force to raise awareness of sickle cell disease and sickle cell trait. The purpose of the Sickle Cell Task Force is to study and advise the department on implementing the recommendations made in the 2018 Sickle Cell Advisory Committee Report published by the Sickle Cell Advisory Committee or any other report the executive commissioner determines is appropriate.
Members
| Name | Membership Category | Public Health Region |
| Presiding Chair Titilope Fasipe, M.D., Ph.D. | Representative of a health-related institution | 6/5 |
| Melissa Frei-Jones, M.D., M.S.C.I. | Physician specializing in hematology | 8 |
| André Harris, M.S.W. | Member of the public who has sickle cell disease (SCD) or is a parent of a person with SCD or sickle cell trait (SCT) | 6/5 |
| Alecia Nero, M.D., M.S.C.S. | Physician specializing in hematology | 2/3 |
| Presiding Vice-Chair Marqué Reed-Shackelford | Member of the public who has SCD or is a parent of a person with SCD or SCT | 6/5 |
| Linda Wade | Member from a community-based organization with experience addressing the needs of individuals with SCD | 7 |
| Vacant | Member from a community-based organization with experience addressing the needs of individuals with SCD | |
| Vacant | Member of the public who has SCD or is a parent of a person with SCD or SCT | |
| Vacant | Texas Education Agency representative | |
| Vacant | Texas Health and Human Services Commission representative | |
| Vacant | Physician with experience addressing the needs of individuals with SCD or SCT | |
| Vacant | Researcher from a public health-related or academic institution with experience addressing SCD or SCT | |
| Vacant | Health care professional with experience addressing the needs of individuals with SCD or SCT |
- Call to order, roll call, and welcoming remarks. The meeting was convened by Linda Wade. A quorum was present.
- Consideration of June 26, 2025, draft meeting minutes. TABLED
- Consideration of draft 2025 SCTF Annual Report
The recommendations from the previous report were continued for the 2025
Sickle Cell Task Force 2024 Annual Report Recommendations
- Evaluate options to increase Medicaid and Children’s Health Insurance Program (CHIP) eligibility for individuals diagnosed with SCD until age 26.
- Use available resources to study development of comprehensive medical home models and ways to create and fund comprehensive sickle cell care centers as a quality improvement project.
- Develop sickle cell quality care plans for Medicaid and private payors.
- Promote incorporating newborn screening trait status checks and follow-up testing into routine adolescent care. {Modify wording significantly)
- Establish a state-level sickle cell quality rating system for health care facilities.
- Collaborate with HHSC to incorporate a reporting process for sickle cell care provided by health care facilities into an existing statewide system.
- Develop partnerships with Texas colleges and universities to create sickle cell awareness campaigns and promote and identify funding for statewide awareness activities.
- Work with the University of Texas School of Public Health in Houston – Center for Health Care Data to develop a sickle cell report from the All-Payor Claims Database. (edit this to include SCDC)
- Collaborate with DSHS to meet the goals of the Centers for Disease Control and Prevention (CDC) Sickle Cell Data Collection (SCDC) program.
- Publish a Texas sickle cell surveillance dashboard to include information from a newly established statewide sickle cell data collection system. (Reword to emphasize Dashboard populated from SCDC)
- The full report may be viewed at: https://www.dshs.texas.gov/sites/default/files/legislative/2024Reports/2024%20Sickle%20Cell%20Task%20Force%20Annual%20Report.pdf
Minor nonsubstantive changes were made to the draft. These included removing age limits and adding working with HHSC to make changes in Medicaid.
MOTION: Adopt the recommendations as edited allowing DSHS and the Chair to make changes prevailed
- Medicaid Cell and Gene Therapy Access Model
- Centers for Medicare and Medicaid Services (CMS) led initiative, state participation
- Focuses on sickle cell disease
- Model framework
- Resources
- CMS Expands Access to Lifesaving Gene Therapies Through Innovative State Agreements
- CMS Cell and Gene Therapy (CGT) Access Model (cms.gov/priorities/innovation/innovation-models/cgt)
Discussion.
Dr. Aliya Ahmad presented updates on the CMS-led Cell and Gene Therapy (CGT) Model for Medicaid. The Model aims to increase access to new gene therapies for sickle cell disease (including Casgevy and Lyfgenia) by reducing costs and using outcome-based agreements.
- Authorized Treatment Center Locator | CASGEVY® (exagamglogene autotemcel)
- LYFGENIA™ (lovotibeglogene autotemcel) | An FDA Approved Gene Therapy for Sickle Cell Disease
The therapy process is complex, involving stem cell collection, manufacturing, infusion, and long-term monitoring at select centers in Dallas, Houston, and San Antonio. Fertility preservation services will be fully covered by manufacturers, a significant addition since Medicaid does not normally cover this.
The model will go live in Texas Medicaid on September 1, 2025, and run through 2035.
Criteria for eligibility and prior authorization are detailed in state manuals and include both a sickle cell diagnosis and further health evaluations.
What are the criteria for the therapy? Diagnosed with sickle cell disease and then further criteria for fitness for stem cell treatment.
A therapy success story was shared by one of the members.
- DSHS Newborn Screening Program updates and announcements. The update was brief.
- Texas newborn screening summit was held and 80 people attend live
- Spoke at the Texas Sickle cell Action initiative
Discussion, No discussion
- Sickle Cell Data Collection Program update. The data from the report were presented. SCDC-THCIC-ED-Report-2023-Final-Draft.pdf. (Texas Health Care Information Collection (THCIC) | Texas DSHS)
| In 2023, the Texas Department of State Health Services (DSHS) received funding from the Centers for Disease Control and Prevention (CDC) to establish Texas SCDC, a state sickle cell data collection system that informs sickle cell disease practices and policies in Texas. Texas SCDC is one of 16 states funded by the CDC. The goal of Texas SCDC is to collect, maintain, and disseminate high quality sickle cell data that will contribute to improving diagnoses, treatments, survival, and quality of life for all individuals with sickle cell disease in Texas.
Texas SCDC provides information on all individuals with sickle cell disease in Texas, regardless of age, insurance status, or geography. Texas SCDC Funding This work was supported by a cooperative agreement from the CDC Sickle Cell Data Collection Program (CDC-RFA-DD230002). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or the U.S. Department of Health and Human Services. |
Sickle Cell Disease Emergency Care In 2023, a total of 19,109 ED visits by individuals with SCD were reported to the THCIC system. The highest rates of ED visits by individuals with SCD in 2023 were in Public Health Region 4 (122 visits per 100,000 individuals) and Public Health Region 5 (145 visits per 100,000 individuals).
Demographics of Sickle Cell Disease Emergency Care
- Black, Non-Hispanic (92.9 percent) was the most common race/ethnicity among ED visits by individuals with SCD in 2023 followed by Hispanic (3.7 percent) and Other, Non-Hispanic (2.1 percent).
- Of the examined age groups, the highest number of ED visits by individuals with SCD in 2023 was by 30- to 39-year-olds (31.6 percent) followed by 20- to 29-year-olds (24.5 percent).
- Children and youth (birth to 24 years old) accounted for 6,065 (31.7 percent) of ED visits by individuals with SCD in 2023.
Reasons and Outcomes for Sickle Cell Disease Emergency Care
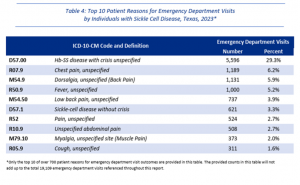
- Hemoglobin SS disease with crisis unspecified (51.2 percent) was the most common principal diagnosis code among ED visits by individuals with SCD in 2023, followed by Sickle-cell disease without crisis (8.3 percent) and Sickle-cell/Hemoglobin C disease unspecified (1.9 percent).
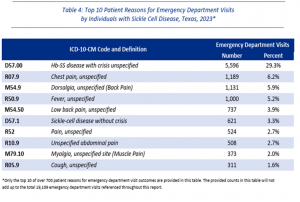
- Most individuals with SCD who visited the ED in 2023 were discharged to home or self-care (91.2 percent), whereas others left against medical advice (5.5 percent) or were discharged to other short term general hospital (1.9 percent).
- In 2023, 10 ED visits by individuals with SCD resulted in death.
Emergency Care Costs
- In 2023, the total costs of ED visits by individuals with SCD in Texas was $175,100,328 and the average cost per ED visit was $9,163.
- The highest cost for an ED visit by an individual with SCD in 2023 was $216,146.
Selected Tables from the Report

Discussion.
Texas SCDC presented key findings from the first SCDC report: 19,109 emergency department (ED) visits for sickle cell disease in Texas (2023 data). Hemoglobin SS (HbSS) with crisis was the most frequent diagnosis (51.2% of principal diagnoses).
Public Health Regions 4 and 5 had the highest ED visit rates. The majority of ED visits were among Black non-Hispanic individuals (92.9%).
- 31.7% of ED visits involved children/youth; 18-24 year-olds were the most frequent youth age group at 41% of youth visits.
- 91.2% of ED visits resulted in discharge home; 1,053 cases left against medical advice; 10 resulted in death.
- Most common reasons for ED visits: sickle cell crisis, chest pain, and back pain (dorsalgia).
- Medicaid was the primary payer for 46.8% of visits; the average visit cost was $9,163 with a total statewide annual ED cost of $175 million.
Data analysis was descriptive, using de-identified and aggregate data, with limitations in tracking repeat visits and certain demographic details. SCDC is planning additional reports (inpatient/non-emergency outpatient visits, hospital-acquired infections) and public outreach (photo blog).
- SCTF subcommittee updates
Medicaid Subcommittee—There has been turnover in the subcommittee. New members have joined. They discussed recommendations in the legislative report. There were a couple of recommendations they came up with. These should be included in the 2025 report discussed above.
- Update TxHealthSteps Education module for Sickle Cell Disease
- Consider adding counseling to periodicity schedule for sickle cell disease
- MCO contract change allowing sickle cell disease
- Pursue Sickle Cell Disease Specific quality metrics
Public Awareness Campaigns Subcommittee— Dr. Nero (with Dr. Reed-Shackleford acknowledged) summarized previous meetings, noting ongoing efforts to reschedule their next meeting. Initial discussions included reviewing school education initiatives for sickle cell, with acknowledgment that some efforts may be duplicative, which was considered positive. The sub-committee is also exploring ways to increase awareness and engagement in sickle cell activities statewide.
Legislatively Mandated Report Subcommittee—Discussed during the report considerations above.
- Sickle Cell Outcomes, Policy, and Excellence Conference. Sickle Cell and Thalassemia Program Sickle Cell Outcomes, Policy and Excellence | Texas Children’s
Dr. Fasipe recapped the conference held in August and attended by approximately 90 participants, mostly in person and some virtually from across Texas and out-of-state.
The event focused on current challenges in sickle cell care, historical context, and paths to improved health outcomes.
- Keynote and panels covered comprehensive care and transition challenges, and advances like transplants and gene therapy.
- There was significant interest in the CMMI model and further engagement is planned.
- Day two centered on Texas priorities, with three workgroups (assurance, assessment, policy development) addressing state-specific sickle cell issues.
- Presentations from the Department of State Health Services and other experts enriched group discussions.
- Legislative engagement was noted, including mention of a new sickle cell registry bill.
- The event was characterized as highly successful with broad task force engagement.
- Texas Sickle Cell Action Plan Initiative Meeting.
This item was covered in item 8 above.
- Public comment. There was no public comment offered.
- Action items and future agenda items. Next meeting is December 12, 2025. Members were invited to propose topics for the next meeting. Members facing scheduling conflicts were asked to contact the task force liaison.
Possible Action Items
- Dr. Fasipe to finalize annual report recommendations text reflecting discussed changes and committee inputs.
- SCDC to move forward with further data analyses and visual outreach materials.
- Medicaid Subcommittee to share detailed recommendations text with Dr. Fasipe for inclusion in the report.
- Public Awareness Campaign subcommittee to plan and execute broader state outreach and collaboration efforts.
- Share eligibility/prior authorization manual links for Medicaid gene therapy with all task force members.
- Adjournment. There being no further business, the meeting was adjourned.
Click HERE to download the full report.
***
The information contained in this publication is the property of Texas Insight and is considered confidential and may contain proprietary information. It is meant solely for the intended recipient. Access to this published information by anyone else is unauthorized unless Texas Insight grants permission. If you are not the intended recipient, any disclosure, copying, distribution or any action taken or omitted in reliance on this is prohibited. The views expressed in this publication are, unless otherwise stated, those of the author and not those of Texas Insight or its management.